Community-based hunting management of large carnivores and herbivores: is a mutually beneficial relationship possible?
By Rachel Skubel, SRC Intern
When conserving species, considering the human dimension is generally essential to a successful trajectory. More and more, as our cities expand on land, and access to the ocean increases, there is inextricable overlap. In some cases, conservation efforts are inherently linked with having these animals around – for example trophy hunting of large carnivores (such as lions in South Africa – although many are captive bred) and herbivores (like the Markhor in Pakistan). This is an important linkage – the large animals of these efforts are often keystone species in the ecosystems they interact with, and can also play an important economic role for human societies they interact with.
What if these large animals disappeared?
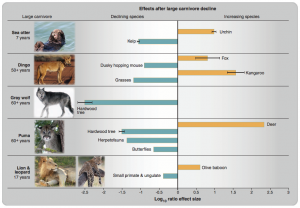
Rather unfortunately, there has been ample opportunity to assess the impacts of large carnivore and herbivore declines on other life. William Ripple has informative publications on both scenarios (herbivores and carnivores). Here are a few examples from Ripple et al. 2015, showing how the loss of one ‘regulating’ species can have cascading impacts on the extent of other species;
Humans <-> Wildlife*
The questions I’m asking today are – outside of fully protected ‘reserves’, which aren’t always feasible, what are some effective conservation strategies? What are some successful programs, and what should be considered going into the future? And, what can be translated from the terrestrial to marine realm?
![Large carnivore and herbivore species are at risk from human activity (Images: National Geographic [http://animals.nationalgeographic.com/animals/wallpaper/lion-stalking-botswana.html], and The Tribune [http://tribune.com.pk/story/968489/big-game-trophy-hunting-helps-revive-markhor-numbers/]](https://sharkresearch.rsmas.miami.edu/wp-content/uploads/2015/11/Figure-2-300x116.png)
Large carnivore and herbivore species are at risk from human activity (Images: National Geographic [http://animals.nationalgeographic.com/animals/wallpaper/lion-stalking-botswana.html], and The Tribune [http://tribune.com.pk/story/968489/big-game-trophy-hunting-helps-revive-markhor-numbers/]
(1) What is the trend of wildlife-human overlap?
In terms of large herbivores, often large protected areas are needed to cover the entire range, for example to account for migrations of wildebeest across the African continent (Boone et al. 2012). Protecting animals from poaching across this massive, dynamic range is a challenge, so this issue persists (Ripple et al. 2015). A study by Rosie Woodroofe (2000) surmises that
“regional and temporal variation in individual species’ sensitivity to human density is more likely to reflect the activities of local people than the phenotypes of local carnivores.”
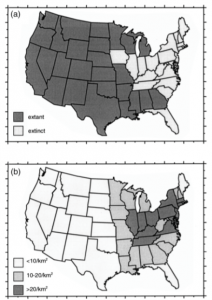
This is neatly visualized by changes in wolf presence in the US from 1900 to 1944 alone (figure 3). Her work analyzes carnivore populations in North and South America, and Africa, to find ‘critical human densities’ (in people/km2) which had already resulted in local extinctions, or may do so in the future based on current trends. These relationships were used to paint a picture of carnivore populations as human density increases.
Woodroofe illustrates that as human density continues to expand, more chances for wildlife conflict will arise. So, how does this conflict relate to social and economic facts?
(2) How do humans perceive the animals?
Some interesting work has been done in Norway on people’s attitudes towards large carnivores. Kleiven et al. (2002) surveyed a representative sample of 3134 individuals, and found that:
- Wolves and bears less acceptable than lynxes and wolverines, when seen close to where the respondents lived
- Acceptability declined with increasing: Lack of personal control, economic loss, and age
- Acceptance was higher among urban residents, and males
The authors’ resulting recommendation was to integrate social and situational (e.g. geographically) predictors of attitudes into wildlife management strategies.
Continuing in this vein, Roskaft et al. 2003 (also Norway) found that people with higher education and an interest in outdoor activities (hiking, hunting) had greater acceptability than those with ‘lower’ education (a measure which ranged from ≤9 to > 12 years of schooling), and no interest in outdoor activities. Accordingly, the authors suggest an educational program about large carnivore ecology (biology and habits), and giving people first-hand experience with the animals.
In a summary of carnivore management over time, Treves and Karanth (2003) describe a transition from fear and economic interests as driving forces, to science-based, ecosystem-based management approaches, which can place less stress on lethal control. The authors recognize the political and social complexities behind effective management, and again stress the importance of engaging the public to enhance conservation success.
To summarize so far, these large animals are increasingly sharing space with humans, and humans are not always accepting of their presence. Aside from habitat destruction, poaching has led to declines in their numbers worldwide, which is unfortunate for both people (i.e. ecosystem services), the species in question, and sometimes for other organisms they indirectly/ indirectly interact with.
(3) Some recent examples of integrating wildlife into human economies
Although these two examples are of different scale, the theme of community-based incentivization of conservation through hunting management is present in both.
One morning on a drive to our field site, my M.Sc. supervisor shared the story of the much-maligned Makhor in Pakistan, which introduced to me the concept of hunting as a conservation strategy. Long subject to poaching, enforcement was difficult over such the Makhor’s large range. In 1994, 1996, and 2008, IUCN red list assessment rated the Makhor as Endangered. However, this animal has transitioned to a sunnier ‘Near Threatened’ status as of 2015, with most subpopulations seeing an increasing trend, and an estimated 5.808 mature individuals in the cumulative population. Although there have been a suite of conservation measures across India, Afghanistan, and Pakistan, the latter has implemented community-based trophy hunting programs in addition to federal protections. Here, the government distributes only 12 hunting permits per year, to these programs. Notably, 80% of the permit fees (when purchased by the eventual hunter) go to the community, and the other 20% to provincial authorities leading conservation efforts [http://www.iucnredlist.org/details/3787/0]. As a result, the community in question is motivated to ensure the health of this species – not only does the permit bring in income year after year, the hunting party might further spend money in the community while in the area (i.e. using local guides). From 1998 to 2008, $830,000 (USD) was allocated to participating communities, and the relative rarity of the permits is increasing their value. However, with only 12 permits available and unmanaged area remaining in the Country, increasing the annual allotment could be the next step.
Trophy hunting in Africa is a multi-faceted topic I can’t possibly cover in its vastness, but is useful to mention as a large-scale contrast to the previous case. An interesting paper from Naidoo et al. (2015) tells us that in Namibia, repurposing agricultural land for both tourism and hunting has provided a relatively consistent income source for the local community, and might provide a strong motivation for conservation. As with our last example, the health of the species can directly influence the health of the local economy, so maintaining the former is of great interest to the community. An overview from Lindsey et al. (2006) tells us a sum of 1,394,000 km2 in sub-Saharan Africa is dedicated to the controversial practice of trophy hunting, incidentally larger than the area of its’ national parks (at the time of the paper). Namibia is among the countries in this region with an increasing number if visiting hunters (1988 – 2006, Lindsey et al. 2006), and has seen an increase in gross annual hunting revenue over the same period. This macro-view is only one side of the story, as socio-economic stability within the related communities, just like conservation success, is dependent on so many factors and measures. As one country in this region prohibits hunting, another may then see an influx of hunters. In areas reliant on income from trophy hunting, there can be a self-sustaining source of money to employ anti-poaching officers, helping to regulate activities detrimental to the target animals and the surrounding community. Photo-tourism is another possible source of income for these hunting areas, and is certainly valuable to consider for countries designing management and conservation plans – are some areas/species better suited to conservation income this source?
With both of these cases, wildlife and humans are linked in their habitats, and in some ways are helping the other persist – and in the case of the Pakistan Markhor, perhaps move away from a trajectory towards local extinction.
Where is the crossover with marine conservation?
Despite the differences in environment and range, as well as stakeholders, there is some common ground with managing large marine species. For example:
- Large sharks, such as white sharks, have faced issues with public perception that challenge their conservation (e.g. Shark culling in Australia – McCaugh et al. 2015). Could social acceptance studies of terrestrial carnivores be used to further understanding of attitudes towards marine predators?
- Design of regulations for mixed-use marine protected areas can draw on lessons from terrestrial areas – what policies that successfully influenced human behavior such that habitat was minimally impacted, which can be translated to a marine environment?
The similarities, as well as the differences, between marine and terrestrial management, (and animal movement) are many. As countries strive to reach their goals of protection in both realms, the crossover between humans and wildlife brings up an important interaction to consider in planning management, and in some cases an opportunity to work towards a mutually beneficial relationship.
References
Boone, R. B., Thirgood, S. J., & Hopcraft, J. G. C. (2006). Serengeti wildebeest migratory patterns modeled from rainfall and new vegetation growth. Ecology, 87(8), 1987-1994.
Gordon, I. J., Hester, A. J., & Festa‐Bianchet, M. (2004). Review: the management of wild large herbivores to meet economic, conservation and environmental objectives. Journal of Applied Ecology, 41(6), 1021-1031.
Kleiven, J., Bjerke, T., & Kaltenborn, B. P. (2004). Factors influencing the social acceptability of large carnivore behaviours. Biodiversity & Conservation, 13(9), 1647-1658.
Lindsey, P. A., Roulet, P. A., & Romanach, S. S. (2007). Economic and conservation significance of the trophy hunting industry in sub-Saharan Africa.Biological conservation, 134(4), 455-469.
McCagh, C., Sneddon, J., & Blache, D. (2015). Killing sharks: The media’s role in public and political response to fatal human–shark interactions. Marine Policy, 62, 271-278.
Naidoo, R., Weaver, C. L., Diggle, R. W., Matongo, G., Stuart‐Hill, G., & Thouless, C. (2015). Complementary benefits of tourism and hunting to communal conservancies in Namibia. Conservation Biology.
Røskaft, E., Bjerke, T., Kaltenborn, B., Linnell, J. D., & Andersen, R. (2003). Patterns of self-reported fear towards large carnivores among the Norwegian public. Evolution and human behavior, 24(3), 184-198.
Ripple, W. J., Estes, J. A., Beschta, R. L., Wilmers, C. C., Ritchie, E. G., Hebblewhite, M., … & Wirsing, A. J. (2014). Status and ecological effects of the world’s largest carnivores. Science, 343(6167), 1241484.
Ripple, W. J., Newsome, T. M., Wolf, C., Dirzo, R., Everatt, K. T., Galetti, M., … & Van Valkenburgh, B. (2015). Collapse of the world’s largest herbivores. Science Advances, 1(4), e1400103.
Treves, A., & Karanth, K. U. (2003). Human‐carnivore conflict and perspectives on carnivore management worldwide. Conservation Biology, 17(6), 1491-1499.
Woodroffe, R. (2000). Predators and people: using human densities to interpret declines of large carnivores. Animal conservation, 3(02), 165-173.