A changing climate impacts fish distributions
By Grace Roskar, RJD Intern
Long-Term Changes in the Distributions of Larval and Adult Fish in the Northeast U.S. Shelf Ecosystem
Fishing pressure and climate change have been linked to changes of the distribution of several fish species in past studies. These factors contributed to geographic shifts of fish populations to different areas and impacted the abundances of adult fish. In this study, researchers at NOAA studied initial stages of fish species’ life cycles to examine how these fish distributions have changed over time and space.
Many fish species switch habitats during different parts of their lives. Most fish species in the Northeast U.S. (NEUS) Shelf Ecosystem produce pelagic eggs. Pelagic eggs float among currents in the open ocean. The eggs hatch into larvae, grow into juveniles, and finally become adults. Since these different life stages often utilize different habitats, at the end of one stage, fish need to be ready to move to a new habitat for the next part of their lives. Past research has focused more on the adult stage than the larval stages. However, the early life stages of fish are critical because a species’ population abundance can be greatly affected by the survival rates of those earlier stages.

This photo shows a larva from the Scombridae family, which includes mackerels, tunas, and bonitos. The Scombridae family was one of fish families sampled by NOAA. Photo credit: NOAA http://www.sefsc.noaa.gov/species/fish/larval/photos_larval.htm
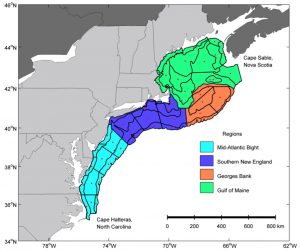
In this study, data from the NOAA Northeast Fisheries Science Center was used to analyze larval distributions in the NEUS Shelf. Past analyses showed that the adult fish distributions in this region have generally shifted towards the north or into deeper water. This study examined two time periods (1977-1987 and 1999-2008). The goals of Walsh et al. (2015) were to “1) examine changes in the spatial distributions of larval fish…2) examine changes in larval seasonal occurrence…3) compare changes in larval distributions to changes in adult distributions, and 4) evaluate changing distributions relative to regional occurrence, timing of larval occurrence, management status of adults, and habitat used by adults.” Larval fish samples were collected from the NEUS Shelf from North Carolina to Nova Scotia by two survey programs. Adult fish samples were collected using bottom trawl surveys over the same area and timeframe.
The results showed that 43% of larval species and 50% of adult species changed distribution. The changes in larval distribution indicate changes in “spawning, currents, survival, or some combination of the three,” (Walsh et al. 2015). This emphasizes the need for further examination into how each stage of the life cycle is connected in these regions. Around 50% of the total taxa exhibited a change in direction, either along the shelf, across the shelf, into deeper waters, such as the movement of Atlantic cod (Gadus morhua), or a combination of these. Most directional changes were towards the north, perhaps an effect of rising temperatures. For 60% of the taxa surveyed, “distribution changes differed between adult and larval stages,” indicating changes in the overall habitat range that species utilize throughout their lives (Walsh et al. 2015). This could be problematic for species if the distribution changes create longer migrations or longer distances between nursery and feeding grounds. These changes in distribution and life stages have significant ramifications for fisheries. When the distribution of a life stage is altered, the stock of the species changes, which can lead to different outcomes for the fishery each year.
Certain off-limits areas in the NEUS Shelf are meant to protect certain species by enforcing fishing regulations. However, when distributions change, the closings could be inadequate at protecting a species if the species simply is not there. Moreover, changes in larval fish distributions can have impacts on the whole ecosystem. For example, if populations are shifting northward, the larvae can be subject to new predators and interactions in the food web. This study showed distributional changes in both larval and adult fish taxa in the NEUS Shelf region. Of importance is that these are complex changes with many factors, such as timing and regional occurrences, which will affect the assessment and management of these resources.
References:
Walsh H.J., Richardson D.E., Marancik K.E., Hare, J.A. 2015. Long-Term Changes in the Distributions of Larval and Adult Fish in the Northeast U.S. Shelf Ecosystem. PLoS ONE 10(9): e0137382. doi:10.1371/journal.pone.0137382